IN NEWS: The Central Drugs Standard Control Organization (CDSCO) recently granted market authorization for NexCAR19, India’s first indigenously developed CAR-T cell therapy, to ImmunoACT, a company incubated by IIT Bombay.
- This paves the way for the commercial launch of this therapy in India, where it is expected to be available to cancer patients at a tenth of the cost abroad.
CAR-T cell therapy
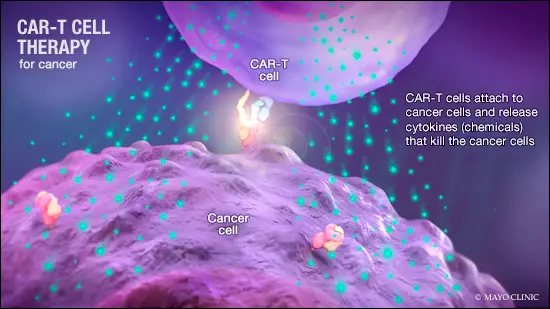
- CAR-T is a revolutionary therapy that modifies immune cells, specifically T-cells, by turning them into potent cancer fighters known as CAR-T cells.
- T-cells are special cells (white blood cells that find and fight illness and infection) whose primary function is cytotoxic, meaning it can kill other cells.
- In CAR-T therapy, we genetically modify them into cancer-fighting cells.
- These supercharged cells are then put back into the body, and they go after cancer cells — especially in blood cancers like leukemia and lymphomas.
NexCar19
- NexCar19 is a type of CAR-T and gene therapy developed indigenously in India by ImmunoACT, which is a company incubated at IIT Bombay.
- Our therapy is designed to target cancer cells that carry the CD19 protein.
- This protein acts like a flag on cancer cells, which allows CAR-T cells to recognise and attach themselves to the cancer cells and start the process of elimination.
- The therapy is for people with B-cell lymphomas who didn’t respond to standard treatments like chemotherapy, leading to relapse or recurrence of the cancer.